MDL 100009
CAS No. 175673-57-1
MDL 100009( —— )
Catalog No. M22953 CAS No. 175673-57-1
MDL 100009, the S-enantiomer of MDL 100151 and the opposite enantiomer of MDL 100907, is a selective antagonist of 5-HT2A.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 462 | In Stock |


|
| 10MG | 669 | In Stock |


|
| 25MG | 1035 | In Stock |


|
| 50MG | 1395 | In Stock |


|
| 100MG | 1872 | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameMDL 100009
-
NoteResearch use only, not for human use.
-
Brief DescriptionMDL 100009, the S-enantiomer of MDL 100151 and the opposite enantiomer of MDL 100907, is a selective antagonist of 5-HT2A.
-
DescriptionMDL 100009, the S-enantiomer of MDL 100151 and the opposite enantiomer of MDL 100907, is a selective antagonist of 5-HT2A.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayEndocrinology/Hormones
-
Target5-HT Receptor
-
Recptor5-HT2A
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number175673-57-1
-
Formula Weight373.5
-
Molecular FormulaC22H28FNO3
-
Purity>98% (HPLC)
-
Solubility——
-
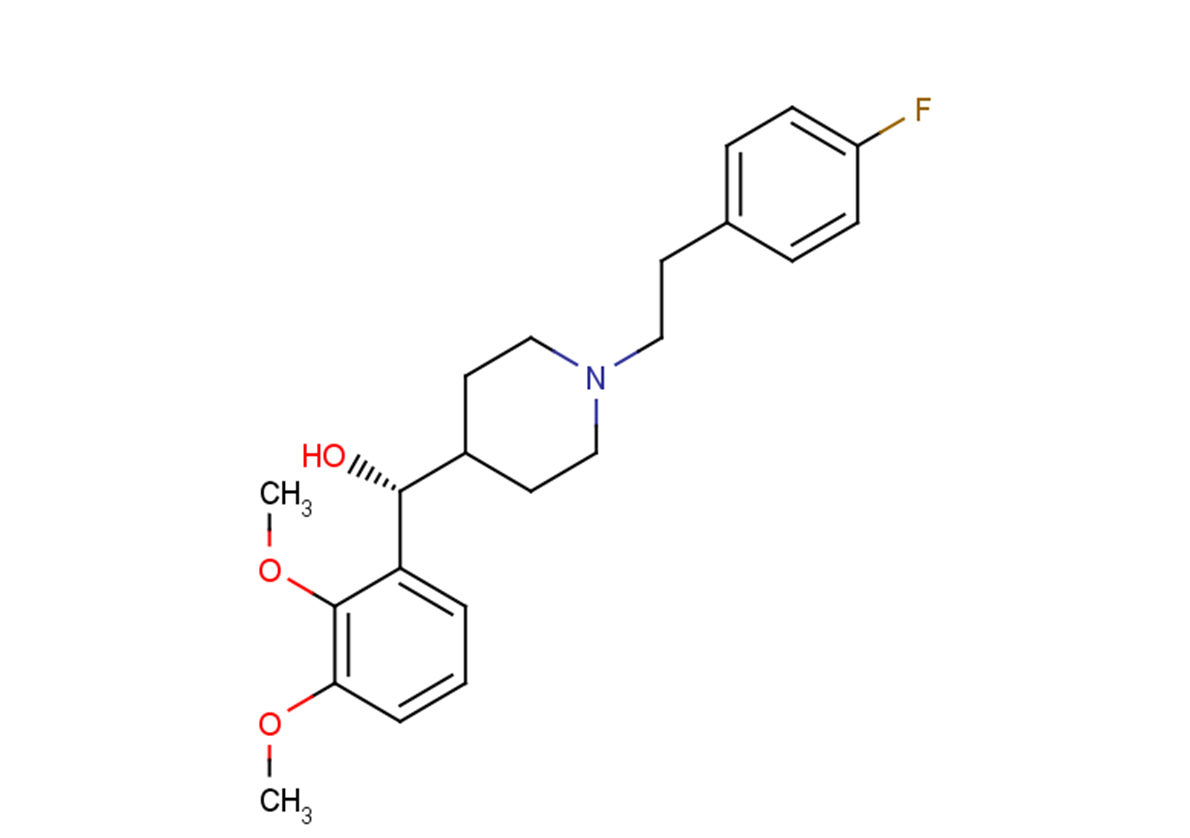
SMILESO[C@@H](C1=CC=CC(OC)=C1OC)C2CCN(CCC3=CC=C(F)C=C3)CC2
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
molnova catalog



related products
-
Ansofaxine hydrochlo...
Ansofaxine (LY03005; LPM570065) is a serotonin-norepinephrine-dopamine reuptake inhibitor (IC50s: 723 763 and 491 nM respectively).
-
m-Chlorophenylbiguan...
m-Chlorophenylbiguanide hydrochloride is a 5-HT3 receptor agonist.
-
Procaine hydrochlori...
A local anesthetic of the ester type that has a slow onset and a short duration of action. It is mainly used for infiltration anesthesia, peripheral nerve block, and spinal block.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


